Acids, Bases and Salts Important Questions Class 7 Science Chapter 5
Acids, Bases and Salts Class 7 Science Chapter 5 Important Questions and Answers are provided here. We prepared these extra questions based on the latest NCERT Class 7 Science Book. These important questions will help you to properly understand a particular concept of the chapter. Practicing class 7 important questions before the exam will help you to get excellent marks in the exam.
Class 7 Science Chapter 5 Acids, Bases and Salts Important Questions
Very Short Answer Type Question
1: The substances used to test whether a substance is acidic or basic are known as ________
Answer: Indicators
2: Name the most commonly used indicator.
Answer: Litmus paper
3: Form where does we extract litmus to be used as indicator.
Answer: Lichens
4: In acidic solution, litmus paper turns into _______
Answer: Red
5: In basic solution, litmus paper turns into _________
Answer: Blue
6: In distilled water, litmus paper turns into _________
Answer: purple
7: The reaction between an acid and a base is known as neutralisation.
Answer: The reaction between an acid and a base is known as neutralisation.
8: Salt and water are produced in neutralisation process with the evolution of ____________
Answer: Heat
9: Name the acid present in our stomach.
Answer: HCL
10: What role does HCL present in our stomach plays?
Answer: It helps in digestion of food
11: Name the acid present in sting of an ant.
Answer: Formic acid
12: State the nature of soap solution.
Answer: Basic
13: State the nature of baking soda.
Answer: Basic
14: State the nature of lemon juice.
Answer: Acidic
15: Why lemon juice and orange juice tastes sour?
Answer: Because they contain acids.
16: Why baking soda taste sour?
Answer: Because it is basic in nature.
17: State one property of acids.
Answer: Acids are sour in taste.
18: State one property of bases.
Answer: Bases are bitter in taste.
19: Tina rubs a solution between fingers and feels soapy, what is the nature of that solution?
Answer: Basic
20: Ammonia is found in many household products, such as window cleaners. It turns red litmus blue. its nature _______________
Answer: Basic
21: The wastes of many factories contain____________
Answer: Acids
22: Blue litmus paper is dipped in a solution. It remains blue, what is the nature of the solution?
Answer: Basic
23: Name the acid that is present in fats in our body.
Answer: Fatty acid
24: Each cell in our body contains an acid, name that acid.
Answer: the deoxyribonucleic acid
25: Hydrogen ion is common to all acids. True/False
Answer: True
26: Name a base that is also used in soda acid fire extinguishers.
Answer: Baking soda.
27: Which gas is liberated when an acid reacts with metals?
Answer: Hydrogen gas
28: The hydrogen ions combine with H2O to form ____________
Answer: hydronium ions (H3O+)
29: Aqueous solution of acid conduct electricity due to ______________ present in it.
Answer: ion
30: What is the common name of the compound CaOCl2?
Answer: Bleaching powder
31: Name a universal indicator.
Answer: pH Scale
32: Define pH.
Answer: pH is the measure of Acidity or Alkalinity of a solution. The term pH stands for negative log of hydrogen ion concentration.
33: The bases that dissolve in water are known as alkalies. True/ False.
Answer: True
34: What is brine?
Answer: Aqueous solution of sodium chloride (NaCl) is known as brine.
35: Phenolphthalein becomes colourless in __________ and pink in_________.
Answer: Colourless in acid and pink in base.
36: What is the colour of methyl orange in acidic solution?
Answer: Pink
37: Name the acid present in vinegar.
Answer: Acetic acid
38: Name the acid present in lemon.
Answer: citric acid
39: Nitric acid turns red litmus blue. True/ False.
Answer: False
40: Tooth decay is caused by the presence of bases. True/ False.
Answer: False
41: Name the acid present in curd.
Answer: Lactic acid
Short Answer Type Questions
1: Give examples of some acids and bases
Answer: Curd, lemon juice, vinegar, orange juice etc. are acids and baking soda, lime water etc. are bases.
2: Define indicators along with examples.
Answer: Indicators are special type of substance that are used to taste whether a substance is acidic or basic in nature. It change the colour of acidic or basis substances when added into it. Turmeric, litmus, etc. are some natural indicators.
3: What is the use of litmus test?
Answer: To test the nature of a substance for an Acid or a base or a neutral, litmus test is performed in which the Acid turns blue litmus red, Bases turn red litmus blue and has no effect on neutral substance.
4: Explain the nature of distilled water.
Answer: The Distilled water is neutral. It is neither sour (Acidic) nor bitter (Basic), to very its chemical nature, we can perform litmus test. It neither turns blue litmus red nor red litmus blue hence we can say, distilled water is a neutral substance.
5: What do you mean by neutral substance, explain with examples?
Answer: The substances which are neither acidic, nor basic are called neutral substance. These substances neither turn blue litmus red nor red litmus blue, for example distilled water, sugar solution etc.
6: Rena is trying to wash turmeric stain on her cloth with soap, she noticed the stain colour changed to red, explain why?
Answer: Turmeric is a natural indicator which when reacts with bases turns it into red colour; here soap solution is basic so it turns red.
7: Red litmus paper is dipped in a solution; it remains red, what is the nature of the solution?
Answer: Red litmus paper when dipped in a solution, if it remains red then the nature of the solution is neutral.
8: Explain the universal indicator.
Answer: Universal indicator gives a range of colour that can be used to determine the level of acidity or basic of a solution, this level is called the pH value.
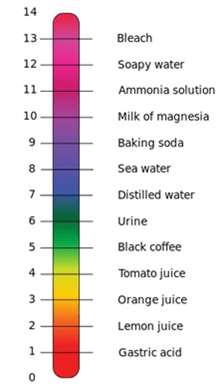
9: A shopkeeper has a few bottles of soft drink in his shop. But, unfortunately, these are not labelled. He has to serve the drinks on the demand of customers. One customer wants acidic drink; another wants basic and third one wants neutral drink. How will he decide which drink is to be served to whom?
Answer: On the basis of the property of acids and bases he can differentiate all the drinks. Like an Acidic drink will have a sour taste and will turn blue litmus red. A basic drink will turn red litmus red. A neutral drink will not change the colour of red or blue litmus. A part from this he can also use turmeric solution for checking the drinks.
10: Why acids do not show acidic behaviour in absence of water?
Answer: Acids do not show acidic behaviour in absence of water because hydrogen ions dissociates from an acid only in the presence of water.
11: Explain why an antacid tablet is taken when we suffer from acidity.
Answer: Sometimes when we consume spicy food, our stomach releases excess of hydrochloric acid which causes indigestion or acidity. An antacid tablets contains base like milk of magnesia (magnesium hydroxide), it neutralises the effect of excessive acid and bring reliefs.
12: Why Calamine solution is applied on the skin when an ant bites?
Answer: The sting of an ant contains formic acid. When an ant bites, it injects the acidic liquid into the skin. The effect of the sting can be neutralised by rubbing some moist solution of a basic substance such as baking soda (sodium hydrogen carbonate) or calamine solution, which contains zinc carbonate.
13: Explain why factory waste should be neutralised before disposing it into the water bodies.
Answer: The wastes of many factories contain acids. If they are allowed to flow into the water bodies, the acids will kill fish and other organisms. The factory wastes are, therefore, neutralised by adding basic substances into it.
14: Define organic acids.
Answer: The acids which are found in plants and animals are called organic acids, for example vinegar contains acetic acid, lemon juice contains citric acid etc.
15: What are salts? Give examples.
Answer: Salts are the ionic compounds generally formed by neutralisation of an acid with base. They can be acidic, basic as well as neutral. Example acidic salts: sodium bicarbonate, basic salts: magnesium chloride, neutral salt: sodium chloride.
16: Give some examples of acidic and basic salt.
Answer: Salts are the ionic compounds generally formed by neutralisation of an acid with base. They can be acidic, basic as well as neutral. Example acidic salts: sodium bicarbonate, basic salts: magnesium chloride, neutral salt: sodium chloride.
17: What do you mean by mixed salt, explain with example?
Answer: A salt derived from more than one acid or more than one base is called mixed salt. Example: bleaching powder, potash alum etc.
Long Answer Type Questions
1: State few properties of acids.
Answer:
- Acids are sour in taste
- The chemical nature of such substances is acidic
- Acid turns blue litmus red
- It gives hydrogen ion when dissolves in water
- Do not give any colour with phenolphthalein indicator
- Do not absorb carbon dioxide gas
- Acids do not react with ammonium salt
- Acids are generally found in Vinegar, Curd, Spinach, lemons, Citrus fruits, Amla, Tamarind, grapes, unripe mangoes, Citrus fruits such as oranges, etc.
2: State few properties of bases.
Answer:
- Bases are bitter in taste and soapy to touch
- Base turns red litmus blue
- The nature of such substances is said to be basic
- It gives hydroxide ions when dissolves in water
- It give pink colour with phenolphthalein indicator
- Some bases like NaOH absorbs carbon dioxide gas
- Bases are generally found in lime water, soap, window cleaner, Milk of Magnesia
- Reacts with ammonium salt to give ammonia gas
3: What are the differences between acids and bases?
Answer:
| Acid | Bases |
| Acids are sour in taste | Bases are bitter in taste and soapy to touch. |
| The chemical nature of such substances is acidic. | The nature of such substances is said to be basic. |
| Acid turns blue litmus red. | Bases turn red litmus blue |
| Acids are generally found in Vinegar, Curd, Spinach, Amla, Citrus fruits, Tamarind, grapes, unripe mangoes, Citrus fruits such as oranges, lemons, etc. | Bases are generally found in lime water, soap, window cleaner, Milk of Magnesia |
| Acids do not react with ammonium salt | Reacts with ammonium salt to give ammonia gas |
| Do not absorb carbon dioxide gas | Some bases like NaOH absorbs carbon dioxide gas |
4: Write a short note about litmus paper.
Answer: Litmus is extracted from lichens. It is most commonly used as an indicator to determine the chemical nature of substance. It has mauve or purple colour in distilled water. When it is added to an acidic solution, it turns red and when added to a basic solution, it turns blue. It is available in the form of a solution, or in the form of strips of paper, known as litmus paper. Generally, it is available as red and blue litmus paper.
5: Arnav is provided with three kinds of liquid of them one is sodium hydroxide; another is hydrochloric acid and third is a sugar solution. How will he identify them when he have only turmeric indicator.
Answer: Turmeric is yellow in colour, when it is exposed to neutral (Sugar Solution) or acidic substances (Hydrochloric Acid) it will retain its yellow colouration. However, if turmeric is exposed to more alkaline substances (sodium hydroxide) it becomes a dark pink/red. So first we detect sodium hydroxide -a basic substance by a colour change from yellow to dark or red. Then will test for an acid or neutral substance with indication of no colour change. Now out of these two, we will mix one with already tested solution for basic substance -sodium hydroxide with dark or red colour, if on mixing the colour reverses back to yellow, the liquid is an acid and the remaining third liquid is neutral.
6: Describe the process of neutralisation with the help of an example.
Answer: Neutralisation is a process in which an acid solution when mixed with base solution, react with each other to produce a salt and water along with generation of heat. Salt so produced, may be acidic, basic or neutral in nature. In this process the acidic nature of the acid and the basic nature of the base are destroyed.
Acid + base salt + water. (heat is evolved)
For example: HCl + NaOH NaCl + H2O.