Extra Questions for Class 7 Science Chapter 6 Physical and Chemical Changes
Extra questions for Class 7 Science Chapter 6 Physical and Chemical Changes with answers is given below. Our subject expert prepared these solutions as per the latest NCERT textbook. These questions will be helpful to revise the all topics and concepts. CBSE Class 7 extra questions are the most simple and conceptual questions that are prepared by subject experts for the students to study well for the final exams. By solving these extra questions, students can be very efficient in their exam preparations.
Physical and Chemical Changes Class 7 Science Extra Questions and Answers
Very Short Extra Questions and Answer
1. Define galvanisation.
Answer: The process of depositing a layer of zinc over iron is called galvanisation.
2. What is the meaning of rusting?
Answer: The formation of rust over the surface of iron is known as rusting.
3. What type of change is crystallisation?
Answer: It is a physical change.
4. What type of change is involved in rusting of iron?
Answer: Rusting of iron is a chemical change.
5. Write one property of stainless steel.
Answer: It does not rust.
6. What type of change is formation of manure?
Answer: Formation of manure is a chemical change.
7. Which of the two is permanent change chemical change or physical change?
Answer: Chemical change is a permanent change.
8. What do you understand by chemical change?
Answer: A change in which one or more new substances are formed is called a chemical change.
9. State the conditions necessary for rusting of iron?
Answer: For rusting, the presence of both oxygen and water (or water vapour) is essential.
10. What is galvanisation?
Answer: The process of depositing a layer of metal like zinc or chromium on iron is called galvanisation.
11. What is added to steel to make it stainless?
Answer: Stainless steel is made by mixing iron with carbon and metals like chromium, nickel and manganese.
Short Extra Questions and Answers
1. Why formation of manure from leaves is a chemical change?
Answer: Formation of manure from leaves is a chemical change because manure formed has a different composition from leaves.
2. Why souring of milk is a chemical change?
Answer: Souring of milk is a chemical change because it results in formation of new substance.
3. What is rust?
Answer: If a piece of iron is left in the open for some time, it acquires a film of brownish substance. This substance is called rust.
4. What is blue vitriol?
Answer: Crystals of copper sulphate pentahydrate are blue in colour. So, it is commonly known as blue vitriol.
5. Name the two methods by which rusting of iron can be prevented.
Answer: Two methods by which rusting of iron can be prevented are by painting or greasing and by galvanizing.
6. What happens when baking soda is treated with vinegar?
Answer: Vinegar (Acetic acid) + Baking soda (Sodium hydrogencarbonate) → Carbon dioxide + other substances
7. Which technique is better for obtaining sugar from sugar solution?
Answer: Crystallization is considered as a better technique for obtaining sugar from sugar solution.
8. What is crystallisation?
Answer: Large crystals of pure substances can be formed from their solutions. The process is called crystallisation.
9. Why a wet iron pan often gets rusted if left in that state for some time?
Answer: A wet iron pan (tawa) often gets rusted if left in that state for some time because iron rust in the presence of oxygen and water.
10. Why does rusting takes place faster during rainy season?
Answer: Rusting takes place faster during rainy season because the content of moisture in air is high in rainy season.
11. What are physical properties?
Answer: Properties such as shape, size, colour and state of a substance are called its physical properties.
12. What is a physical change?
Answer: A change in which a substance undergoes a change in its physical properties is called a physical change.
13. What is called a chemical reaction?
Answer: A change in which one or more new substances are formed is called a chemical change. A chemical change is also called a chemical reaction.
14. Why a slice of an apple acquires a brown colour if it is not consumed immediately?
Answer: A slice of an apple acquires a brown colour if it is not consumed immediately due to the formation of new substances.
15. Write down the equation representing the process of rusting.
Answer: The process of rusting can be represented by the following equation:
Iron (Fe) + Oxygen (O2, from the air) + water (H2O) → rust (iron oxide Fe2O3)
16. Why tearing of paper into pieces is a physical change?
Answer: Tearing of a paper is a physical change because when the paper is torn only the shape and size of the paper is changed, no new substance is formed.
17. What happens when magnesium oxide is dissolved in water?
Answer: On dissolving the magnesium oxide in water it forms a new substance. This change can be written in the form of the following equation:
Magnesium oxide (MgO) + Water (H2O) → Magnesium hydroxide [Mg(OH)2]
18. Why is spoiling of food a chemical change?
Answer: Spoiling of food is an unwanted quality change in a foodstuff, such as staling, discoloration, the development of off-flavours and odours. It is a chemical change as it cannot be brought back to its original form.
19. How would you show that setting of curd is a chemical change?
Answer: Setting of curd is a chemical change because
- Once curd is formed it cannot be reversed back into milk.
- Curd and milk have different properties.
20. Why explosion of fireworks is a chemical change?
Answer: Explosion of a firework is a chemical change because explosion produces heat, light, sound and unpleasant gases and once the crackers are burnt, it cannot be brought back to its original form.
21. Why is hacksaw blade’s color change on heating considered as a physical change?
Answer: Changing of hacksaw blade’s color on heating is considered as a physical change because in this process only colour changes, no new substance is formed.
22. Why do ships suffer a lot of damage from rusting in spite of being painted?
Answer: Ships suffer a lot of damage from rusting in spite of being painted because the water of the sea contains many salts which makes the process of rust formation faster.
Long Extra Questions and Answers
1. Explain how painting of an iron gate prevents it from rusting.
Answer: For rusting, the presence of both oxygen and water (or water vapour) is essential. Painting of an iron gate prevents it from coming in contact with oxygen, or water, or both and thus prevents it from rusting.
2. Why is melting of ice a physical change?
Answer: Melting of ice is a physical change because it causes change in the physical state of water i.e., from ice in the solid state to water in the liquid state. Moreover, no new substance is formed in the process and this change is reversible. So, melting of ice is a physical change.
3. What happens when carbon dioxide is passed through lime water?
Answer: When carbon dioxide is passed through lime water, calcium carbonate is formed, which makes lime water milky.
Carbon dioxide (CO2) + Lime water [Ca(OH)2] → Calcium Carbonate (CaCO3) + Water (H2O)
4. Why rusting of iron objects are faster in coastal areas than in deserts?
Answer: For rusting, the presence of both oxygen and water (or water vapour) is essential. In coastal areas moisture present in the air is higher compared to desert areas. Thus, rusting of iron objects is faster in coastal areas than in deserts.
5. Why stretching of rubber band is a physical change?
Answer: When rubber band is stretched only its size changes and it comes back in its original shape and size, once it is released. Moreover, it does not cause any change in its chemical composition. Hence, stretching of rubber band is a physical change.
6. Ozone acts as a natural shield against ultraviolet radiation. Explain
Answer: It protects us from the harmful ultraviolet radiations which come from the sun. Ozone absorbs this radiation and breaks down to oxygen. If ultraviolet radiation were not absorbed by ozone, it would reach the earth’s surface and cause harm to us and other life forms.
7. What happens when magnesium ribbon is burnt in air?
Answer: When the magnesium ribbon burnt in air, it reacts with oxygen found in the air to form Magnesium Oxide. After it burns, it forms a white powder of the magnesium oxide. The change can be represented by the following equation:
Magnesium (Mg) + Oxygen (O2) → Magnesium oxide (MgO)
8. What is rusting?
Answer: If a piece of iron is left in the open for some time, it acquires a film of brownish substance. This substance is called rust the process is called rusting.
The process of rusting can be represented by the following equation:
Iron (Fe) + Oxygen (O2, from the air) + water (H2O) → rust (iron oxide Fe2O3)
9. When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it? Explain.
Answer: When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas.
We can write the reaction as:
Lemon juice (Citric acid) + Baking soda (Sodium hydrogencarbonate) → Carbon dioxide + other substances
It is a chemical change.
10. Why are chemical changes important in our daily lives?
Answer: Chemical changes are very important in our lives because all new substances are formed as a result of chemical changes. For example, if a metal is to be extracted from an ore, such as iron from iron ore, we need to carry out a series of chemical changes. A medicine is the end product of a chain of chemical reactions. Useful new materials, such as plastics and detergents, are produced by chemical reactions. Indeed, every new material is discovered by studying chemical changes.
11. In addition to new products, what else may accompany a chemical change?
Answer: In addition to new products, the following may accompany a chemical change:
- Heat, light or any other radiation (ultraviolet, for example) may be given off or absorbed.
- Sound may be produced.
- A change in smell may take place or a new smell may be given off.
- A colour change may take place.
- A gas may be formed.
12. Classify the changes involved in the following processes as physical or chemical changes:
Answer: (a) Photosynthesis – chemical change
(b) Dissolving sugar in water – physical change
(c) Burning of coal – chemical change
(d) Melting of wax – physical change
(e) Beating aluminium to make aluminium foil – physical change
(f) Digestion of food – chemical change
(g) Condensation of steam – physical change
(h) Spoiling of food – chemical change
(i) Burning of coal – chemical change
13. When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
Answer: When a candle burns, both physical and chemical changes take place.
The melting of the solid wax to form liquid wax and the evaporation of liquid wax to form wax vapour are physical changes. The burning of the wax vapour is a chemical change.
Example
Burning of LPG involves both physical and chemical changes because when LPG comes out of cylinder and is converted from liquid to gaseous state it is a physical change. When the gas burns in air it is a chemical change.
14. Explain why burning of wood and cutting it into small pieces are considered as two different types of changes.
Answer: Burning of wood
Burning is a non-reversible chemical change because when we burn wood new substances are formed as the carbon in the wood reacts with oxygen in the air to create ash and smoke, and energy in the form of light and heat.
Cutting of wood it into small pieces
Cutting of wood into small pieces are physical change as no new substance is formed. Only shape and size changes when wood is cut into small pieces.
15. What happens when an iron nail is dipped in copper sulphate solution?
Answer: When an iron nail is dipped in the copper sulphate solution (CuSO4) then iron displaces copper from the copper sulphate because iron is more reactive than copper. The change of colour of the solution from blue to green is due to the formation of iron sulphate (FeSO4), a new substance. The brown deposit on the iron nail is copper, another new substance.
We can write the reaction as:
Copper sulphate solution (blue) + Iron → Iron sulphate solution (green) + Copper (brown deposit)
16. Differentiate between physical and chemical change.
Answer:
| Physical Change | Chemical Change |
| 1. Physical change effect only physical properties. | 1. Chemical change effect both physical and chemical properties. |
| 2. Physical change is generally reversible. | 2. Chemical change is irreversible. |
| 3. In such a change no new substance is formed. | 3. In such a change new substance is formed. |
| 4. Example: Condensation of steam | 4. Example: Burning of coal |
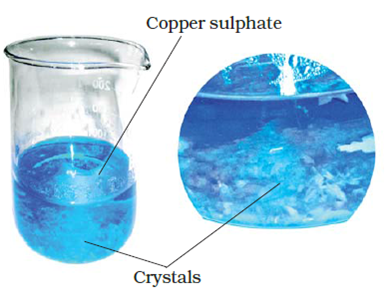
17. Describe how crystals of copper sulphate are prepared.
Answer: Take a cupful of water in a beaker and add a few drops of dilute sulphuric acid. Heat the water. When it starts boiling add copper sulphate powder slowly while stirring continuously. Continue adding copper sulphate powder till no more powder can be dissolved. Filter the solution. Allow it to cool. Do not disturb the solution when it is cooling. Look at the solution after some time. You see the crystals of copper sulphate.