Important Questions for Class 6 Science Chapter 4 Sorting Materials into Groups
Important Questions for Class 6 Science Chapter 4 Sorting Materials into Groups is given in this article. The answers of these important questions is prepared by our expert teachers as per the latest NCERT book and CBSE guidelines. Practicing these questions before the exam will help students to get excellent marks in the exam. Students can also download PDF of Class 6 Science Chapter 4 Sorting Materials into Groups important questions and answers from the links below.
Sorting Materials into Groups Class 6 Important Questions and Answers
Below we have complied the Class 6 Science Chapter 4 Sorting Materials into Groups important questions with answers. These important questions are divided into three parts. They are – Very short questions, short questions and long important question. CBSE important Questions for Class 6 Science will help to score more marks in your CBSE Board Exams.
Very Short Answer Type Questions
1. Why do we need to group materials? Give one reason.
Answer: We often group materials for our convenience. It helps to describe their properties.
2. Suggest two bases on which we can group objects.
Answer:
(i) Material used in making the object, e.g. wood or metal/plastic.
(ii) Material of the object is soft or hard, or substance is soluble or insoluble in water.
3. Is a substance which can be compressed soft or hard?
Answer: Soft.
4. Select a lustrous material out of the following substances:
Answer: Aluminium.
5. Which material is generally used for making pens? Wood, aluminium, plastic, cotton
Answer: Plastic or metal.
6. Is oil soluble in water?
Answer: Oil does not dissolve in water, so it is insoluble in water but floats on the surface of water.
7. Name two objects which are made from opaque materials.
Answer: Wooden doors, blackboard/steel plate.
8. What is common between salt and sand?
Answer: Both have mass and are in solid state.
9. List three liquids which are transparent.
Ans. Water, alcohol and Acetone/Benzene.
10. Write two substances which are made from leather.
Answer: Belt and shoes.
11. Name some substances which are made from plastics.
Answer: Toys, plates, cups, buckets, baskets.
12. Which is more hard, sponge or iron?
Answer: Iron is harder than sponge.
13. Write two gases which are soluble in water.
Answer: Oxygen, Carbon dioxide.
14. Name two gases which are insoluble in water.
Answer: Hydrogen and Nitrogen.
Short Answer Type Questions
1. Write any four properties of materials.
Answer:
(a) Appearance
(b) Hardness
(c) Solubility
(d) Float or sink in water
(e) Transparency
2. Why is a tumbler not made with a piece of cloth?
Answer: We use tumblers made of glass, plastic and metal to keep a liquid. These substances can hold a liquid.
A tumbler made of cloth cannot hold a liquid because:
(i) Cloth piece is not hard enough to hold liquids and
(ii) Cloth piece has very minute pores through which the liquid oozes out.
3. What are the similarities between iron, copper and aluminium?
Answer:
(a) They all have lustre,
(b) They are all metals,
(c) They are hard.
4. Mention some materials which are made up of paper.
Answer: Books, notebooks, newspapers, toys, calendars, etc.
5. Why is water important for our body?
Answer: Water can dissolve a large number of substances, so it is needed by the body. It is also major part of our body cells.
6. What is the basis for sorting materials?
Answer: Materials are grouped on the basis of similarities or dissimilarities in their properties.
7. What is the reason for grouping materials?
Answer: Materials are grouped for our convenience to study their properties and also observe any patterns in these properties.
8. Metals have lustre (shine). Give reason why some metal articles become dull and lose their shine.
Answer: Metals when exposed to air react with moisture and gases present in it, thereby forming a dull layer of some other compound on it.
9. Kerosene, coconut oil, mustard oil do not dissolve in water, even on shaking. They separate after sometime forming two different layer. Explain why.
Answer: The molecules of water do not intermingle (mix) with the molecules of oil. The space between the molecules of water is not taken by oil, so they are immiscible in water.
10. Name a non-metal that has lustre.
Answer: Iodine.
11. Metals generally occur in solid state and are hard. Name a metal that exists in liquid state and a metal that is soft and can be cut with knife.
Answer: Mercury is a metal that exists in liquid state. Sodium and Potassium are soft metals and can be cut with knife.
15. Name the naturally occurring hardest substance known.
Answer: Diamond, it is made up of carbon (non-metal).
16. Why is water called a universal solvent?
Answer: Water dissolves a large number of substances in it. So it is called universal solvent.
Long Answer Type Questions
1. ‘Grouping of objects helps the shopkeeper.’ Justify the statement.
Answer: Proper grouping of objects helps shopkeeper in the following ways:
(i) He can locate the required object easily and quickly.
(ii) He can easily come to know what stocks are going to finish and he should purchase them for his customers.
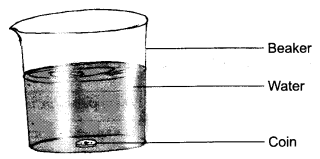
2. Describe an experiment to prove that water is transparent.
Answer: Take a beaker half-filled with clean water. Put a coin in beaker of water.
Place the beaker undisturbed for a few minutes where enough light is present. Now, observe the coin immersed in water from the top of the beaker. Are you able to see the coin? You can clearly see the coin immersed in water. This proves that water is a transparent liquid.
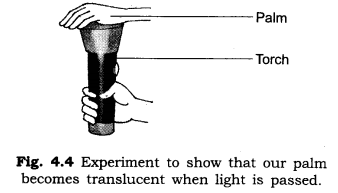
3. Write an experiment to show that our palm is translucent.
Answer: Cover the glass of a torch with your palm at a dark place. Switch on the torch and observe from the other side of palm. We see that the light of torch passes through palm but not clearly. This experiment shows that our palm becomes translucent when a strong beam of light passes through it.
4. How can you show that some solids like sugar, salt are soluble in water whereas solids like chalk powder and sand are not soluble in water?
Answer: Collect samples of sugar, salt, chalk powder and sand. Take four beakers. Fill each one of them about two-third with water. Add a teaspoonful of sugar to the first beaker, salt to the second, chalk powder to the third and sand to the fourth. Stir the contents of each beaker with a spoon/stirrer.
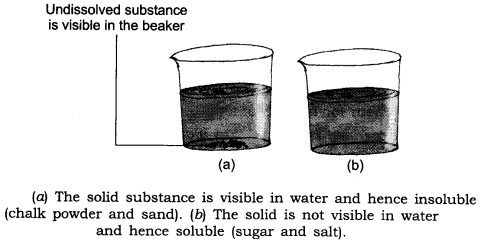
Wait for a few minutes and observe what happens to the substances added to the’ water.
Note down your observations in the following table.
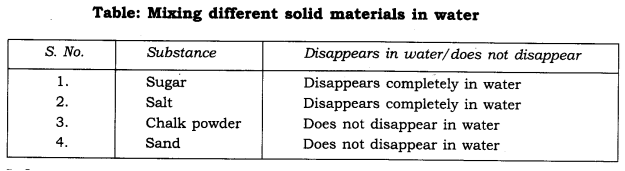
Inference:
(i) Sugar and salt are soluble in water.
(ii) Chalk powder and sand are insoluble in water.