Metals and Non-metals Class 10 Important Questions and Answers
Important Questions for Class 10 Science Chapter 3 Metals and Non-metals covers each topic of the chapter. These questions aim at providing a better understanding of the chapter to the students and can be downloaded in PDF format. These important question bank help students in clearing their doubts so that they can score well in the exam.
While preparing for exams, students should practise these important questions of Class 10 Science to understand the concepts better. Solving important questions of Class 10 Science Chapter 3 will teach students time management skills and enhance their problem-solving skills. Also, students may come across a few of these questions in the board exam.
Important Questions for Class 10 Science Chapter 3 – PDF
1. Name one metal and one non-metal that exists in liquid state at room temperature.
Answer: Mercury is a metal that exists in liquid state at room temperature. Bromine is the non-metal that exists in liquid state at room temperature.
2. Why metals can act as reducing agents while non-metal cannot?
Answer: Metals act as reducing agents because highly reactive metals have the ability to displace low reactivity metals from their compounds. Non-metals do not display this property.
3. What type of oxides are formed by?
(a) Metals
(b) Non-metals?
Answer: (a) Metals form oxides (metallic oxides) which are basic in nature. Some examples are Na2O, K2O etc.
(b) Non-metals form oxides (non-metallic oxides) which are acidic in nature. Some non-metallic oxides are NO2, SO2.
4. Name two non-metals which are lustrous.
Answer: Iodine and carbon are non-metals which are lustrous. Note that carbon is lustrous only in certain forms like diamond, and graphite.
5. Name two metals:
(a) which have very low melting points,
(b) which are so soft that they can be cut with a knife.
Answer: (a) Gallium and Caesium are two metals which have very low melting points. The melting point is so low that they start to melt if kept in our palms.
(b) Sodium and potassium are two metals which are soft and can be cut with a knife. They belong to the group of alkali metals. (Group 1 elements)
6. Name two metals:
(a) which are best conductors of heat,
(b) which are comparatively poor conductors of heat.
Answer: (a) Silver and copper are the best conductors of heat.
(b) Mercury and lead are two poor conductors of heat.
7. What is meant by malleability? Name two most malleable metals.
Answer: The property of metals which allow them to be beaten into thin sheets is called malleability. Gold and silver are the most malleable metals.
8. What is meant by ductility? Name two most ductile metals.
Answer: The ability of the metals to be drawn into thin wires is known as ductility. Gold and platinum are the most ductile metals.
9. Name two metal oxides which are:
(a) water soluble
(b) insoluble in water.
Answer: (a) Sodium oxide (Na2O), potassium oxide (K2O) are metal oxides which are water soluble.
(b) Copper (II) oxide (Cu2O), Beryllium oxide (BeO) are metal oxides which are water insoluble.
10. What is meant by anodising? Why is it done?
Answer: The process of coating an oxide layer over a metal is called anodizing. Usually, aluminium is selected as the metal, and the oxide coating will be aluminium oxide. Anodising is done to make the metal resistant to further corrosion.
11. Write the electron-dot structure for Na and Cl atoms. How do these form a chemical bond? Name the type of bond so formed. Why does a compound so formed have high melting point?
Answer: The reactivity of the elements is determined by the tendency to have a completely filled valence shell. The electron dot structure of Na (sodium) and Cl (chlorine) atoms are shown in the figure below.

To attain completely filled valence shell, Na has to lose the outermost electron, and Cl needs one electron to complete the valence shell.
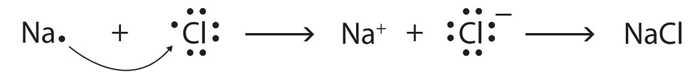
By losing an electron, Na becomes Na+ (cation) and this electron is accepted by Cl to form Cl- (anion). The oppositely charged ions attract each other and form NaCl. They are held together by strong electrostatic forces of attraction. The bond existing between them is called an ionic bond.
Since the inter-ionic forces are very strong, it requires a considerably large amount of energy to break the bond. Hence, ionic compounds have high melting point.
12. Differentiate between metals and non-metals with respect to their chemical properties.
Answer: The properties of metals and non-metals are given in the form of a table shown below. The properties mentioned here apply to the metals and non-metals generally. However, all metals and non-metals may not obey the given properties; there might be exceptions.
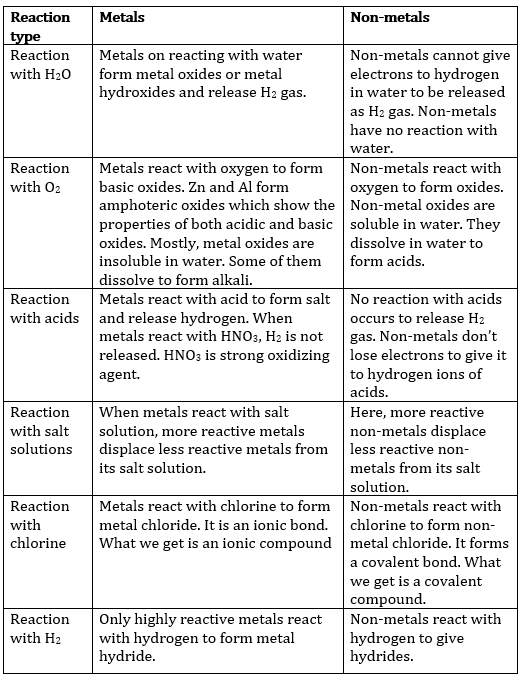
13. Differentiate between roasting and calcination.
Answer: The process of heating a sulphide ore strongly in the presence of excess air is called roasting.
The process of heating a carbonate ore strongly in the presence of limited air is called calcination.
14. Define the following terms:
(a) Alloy
(b) Corrosion.
Answer: (a) An alloy is a homogenous mixture of two or more metals, mixed in a suitable proportion.
(b) Corrosion is the process of destruction of a material caused by chemical reactions between the material and the surrounding environment.
15. What happens when:
(a) iron reacts with steam?
(b) calcium and potassium are put in water?
Answer: (a) Iron (II) oxide (FeO), as well as iron (III) oxide (Fe2O3), are oxides of iron. When iron reacts with steam, both oxides are formed and the product is in a combined form (FeO.Fe2O3 or Fe3O4).
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
(b) Calcium and potassium are two highly reactive metals in the reactivity series.
Potassium reacts very violently with water, and hydrogen is released. The reaction is so exothermic that the evolved hydrogen catches fire immediately.
2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) + heat energy
The reaction of calcium with water is not as violent as the above reaction. The heat evolved is not sufficient for the hydrogen to catch fire. Also, calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of calcium.
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
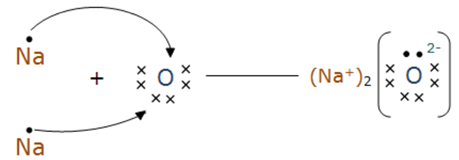
16. Show the formation of:
(a) MgO
(b) Na2O by the transfer of electrons.
Answer: (a) Magnesium loses two electrons to attain a noble gas configuration, whereas oxygen gains two electrons to completely fill the valence shell.
Mg → Mg2+ + 2e–
O + 2e– → O2–
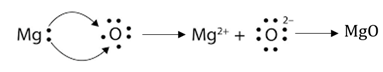
(b) Sodium loses one electron to have a completely filled valence shell, whereas oxygen gains two electrons to complete the valence shell.
Na → Na+ + e–
O + 2e– → O2–
17. Why do ionic compounds:
(a) are hard and solids?
(b) conduct electricity in their aqueous solution form or molten state?
Answer: (a) Ionic compounds are made up of oppositely charged ions which are held together by strong electrostatic force of attraction. Due to this reason, they are hard solids. When large pressure is applied, they tend to break into pieces.
(b) Conduction of electricity is achieved by the movement of charged particles. In the molten state or in their aqueous solution form, ionic compounds contain ions which can move throughout the solution/molten solid, which helps in conducting electricity.
18. How are the following metals reduced:
(a) Metals which are highly reactive?
(b) Metals which are in the middle of the reactivity series?
Answer: (a) Highly reactive metals (e.g., sodium, potassium) are reduced from their compounds by electrolytic reduction. By electrolyzing the chlorides or oxides of these metals, the pure metal is deposited at the cathode.
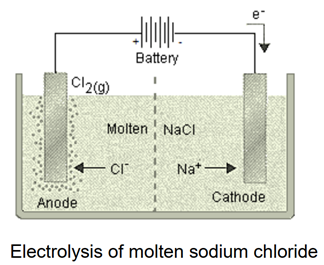
(b) Metals which come in the middle of the reactivity series are reduced from their compound using any of the following techniques.
- If the compound is a metal oxide, then it is reduced to the corresponding metals by reduction using carbon (coke) in the presence of heat.
- If the compound is a metal carbonate, then it is converted into corresponding metal oxide by strongly heating it in the limited air (calcination). Later, metal oxides are reduced to metals using carbon.
- If the compound is a metal sulphide, then it is converted into corresponding metal oxide by strongly heating it in excess air (roasting). Then the metal oxide obtained is reduced to metal using carbon.
19. How you will obtain the following metals:
(a) mercury from cinnabar?
(b) Copper from its ore copper glance?
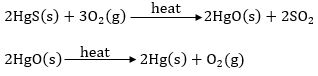
Answer: (a) Cinnabar (HgS – mercury (II) sulphide or mercuric sulphide) is an ore of mercury. It is heated in air to give mercuric oxide (HgO). Mercuric oxide is further heated to get mercury. The reactions involved are,
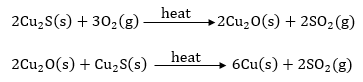
(b) Copper glance is a sulphide ore of copper (Cu2S). So it is converted to cuprous oxide (Cu2O) by just heating in air. Cuprous oxide is reduced to copper using carbon. The reactions involved are:
20. What happens when:
(a) manganese dioxide is heated with aluminium powder?
(b) iron (III) oxide is heated with aluminium powder?
Answer: (a) When manganese dioxide (MnO2) is heated with aluminium powder, displacement reaction takes place, and manganese is obtained as the product along with aluminium oxide. It is an exothermic reaction and hence Mn is obtained in molten form.
3MnO2(s) + 4Al(s) → 3Mn(l) + 2Al2O3(s) + heat
(b) Iron (III) oxide is heated with aluminium powder to form iron and aluminium oxide. This is another example of a displacement reaction and is highly exothermic. This reaction is also known as thermit reaction.
Fe2O3(s) + 2Al(s) → 2Fe(l) + Al2O3(s) + Heat
21. How will you extract metal M from its enriched sulphide ore, if M is in the middle of the reactivity series? Write various steps used in extracting this metal.
Answer: For a metal in the middle of the reactivity series, having a sulphide ore, the following processes are done to extract the metal M.
(i) The ore might contain impurities (also called gangue) which must be removed before the extraction of metal.
(ii) The metal sulphide ore is subjected to strong heating in excess air. This process is called roasting, and it converts the metal sulphide to the corresponding metal oxide. The reaction can be represented as:
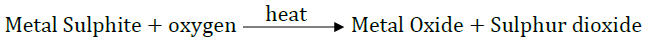
(iii) Now, the metal oxide is reduced to the metal M using suitable reducing agents like C (coke) in the presence of heat.
Metal oxide + C → M + CO
(iv) Once the extraction is complete, M is subjected to various refining processes to remove impurities from the metal.
22. What is a reactivity series? Describe an activity to develop a reactivity series.
Answer: A reactivity series is a list of metals which are arranged in the order of their decreasing activity. This means the metal at the top of the series is the most reactive and the metal at the bottom of the list is the least reactive metal.
To develop a reactivity series, take pieces of different metals (e.g., iron, copper, zinc etc) and the salt solutions of those metals (i.e., iron sulphate, copper sulphate, zinc sulphate etc).
Add a metal piece to a different metal salt solution. Check for any colour changes in the solution or to the metal piece and record your observations.
One can tell that for metals A and B if the following reaction occurs,
Metal A + salt solution of B → metal B + salt solution of A
Then, metal A is more reactive than B. Similarly by trying out various combinations of metals with metal salt solutions, one can create a reactivity series.
23. How electrolytic refining of copper is carried out? Explain in detail.
Answer: The electrolytic refining of copper is done using the apparatus shown in the figure below.
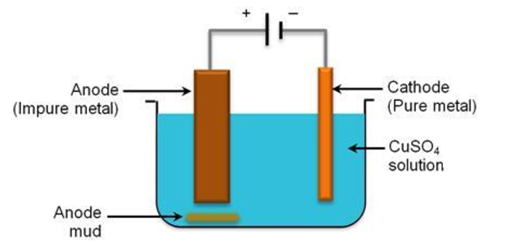
This is a standard electrolysis setup, where the impure copper (the sample to be refined) is placed as anode and a thin strip of pure copper is placed as cathode. The electrolyte used is copper sulphate solution. When current is passed through the electrolyte, pure copper content from anode dissolves into the electrolyte, and the same amount of copper is deposited on the cathode. One can see that the thickness of the cathode increases.
The soluble impurities present in the anode goes into the solution when the current flows through the setup. The insoluble impurities present in the anode settle down at the bottom of the anode and is called anode mud. Thus, one can refine copper electrolytically.
24. Define the following:
(a) Amalgam
(b) Ore
(c) Mineral.
Answer: (a) An alloy of mercury with another metal is called amalgam.
(b) Mineral which contains a very high percentage of a metal and from which the metal can be economically extracted is called an ore.
(c) An element or compound which occurs naturally in the Earth’s crust is called a mineral.
25. What is meant by corrosion? How corrosion is caused? How it can be prevented? What is the effect of corrosion on:
(a) copper?
(b) silver?
Answer: Corrosion refers to the destruction or deterioration of a substance due to its interactions with the surrounding environment. Corrosion is a natural process and occurs when the substance is in contact with air, water, chemicals like acid etc. Corrosion can damage metals causing it to lose properties such as strength and conductivity of heat/electricity.
Corrosion can be prevented by various techniques like painting the surface of the material, galvanizing, oiling, greasing etc.
(a) Copper when in contact with moist carbon dioxide in air gains a green coloured coat over copper. This coat is nothing but copper carbonate formed by the reaction of copper and moist carbon dioxide.
(b) Silver reacts with sulphur present in the air to form a black coating of silver sulphide over the surface of silver.
26. Give reasons only:
(a) Na, K and Li are stored under oil.
(b) Pt, Au and Ag are used to make jewellery.
(c) Hydrogen gas is not evolved when metals react with nitric acid.
(d) Aluminium is a reactive metal, yet it is used to make utensils for cooking.
(e) Aqueous copper sulphate solution should not be stored in an iron container.
Answer: (a) Na, K and Li react vigorously with air and water. Hence they cannot be kept in open or in water. They do not react with oil or kerosene. They are stored under oil to avoid contact with air and water.
(b) Pt, Au and Ag are lustrous. They shine brightly when light falls on them. Besides, these metals are easily malleable and ductile to make chains, rings, and so on. Thus they are used in jewellery.
(c) Nitric acid is a strong oxidizing agent. The hydrogen gas obtained from the reaction is quickly oxidized to water (H2O).
(d) Aluminium is a light metal. It is a good conductor of heat. It has a relatively high melting point. Due to the reactivity of aluminium, it combines with oxygen to form a layer of aluminium oxide over the surface of aluminium. This oxide layer prevents it from further reactivity of the metal. All these reasons make aluminium useful in making cooking utensils.
(e) Iron is more reactive than copper. So iron would displace copper from its salt solutions. In this case, the following reaction will occur:
Fe + CuSO4→ FeSO4 + Cu
Hence, there will no longer be copper sulphate solution in the container. It is advised to store chemicals with containers made of less reactive metals.