NCERT Solutions for Class 8 Science Chapter 14 Chemical Effect of Electric Current
NCERT Solutions for Class 8 Science Chapter 14 Chemical Effect of Electric Current are given below. Here we have provided the best and error-free answers to all the exercise questions that will strengthen your foundation in science. Solving NCERT questions will assist you in grasping the content in the Crop Production and Management chapter in a better way.
In these solutions, we have answered all the intext and exercise questions provided in NCERT class 8 science textbook. NCERT Solutions for Class 8 Science Chapter 14 Chemical Effect of Electric Current provided in this article are strictly based on the CBSE syllabus and curriculum. Students can easily download these solutions in PDF format for free or can read them online.
Chemical Effect of Electric Current Class 8 Science NCERT Solutions
Exercise Questions
Question 1: Fill in the blanks
(a) Most liquids that conduct electricity are solutions of , ______________ and ______________.
(b) The passage of an electric current through a solution causes ______________ effects.
(c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the ___________terminal of the battery.
(d) The process of depositing a layer of any desired metal on another material by means of electricity is called _________.
Answer: (a) Most liquids that conduct electricity are solutions of acids, bases and salts.
(b) The passage of an electric current through a solution causes chemical effects.
(c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the negative terminal of the battery.
(d) The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
Question 2: When the free ends of a tester are dipped into a solution, the magnetic needle shows deflection. Can you explain the reason?
Answer: The deflection in the compass needle shows that current is flowing through the wounded wire and hence, through the circuit. The circuit is complete since free ends of the tester are dipped in a solution. The solution is certainly a conducting solution. This is the reason why the compass needle shows a deflection.
Question 3: Name three liquids, which when tested in the manner shown in Fig.14.9, may cause the magnetic needle to deflect.
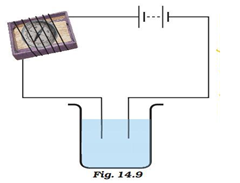
Answer: Ground water, vinegar, citric fruit juice. The liquid solutions containing salts (basic or acidic) will conduct electricity.
Question 4: The bulb does not glow in the setup shown in Fig.14.10. List the possible reasons. Explain your answer.
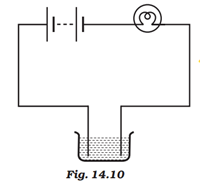
Answer: The possibility of the bulb not glowing maybe because of the following reasons:
(a) The liquid may be non-conducting. In this case, the circuit is incomplete and the current does not pass through the liquid.
(b) Electric current may be weak for the circuit is made up of a material which is not a good conductor of electricity or there is insufficient energy in the battery to generate electricity.
Question 5: A tester is used to check the conduction of electricity through two liquids, labelled A and B. It is found that the bulb of the tester glows brightly for liquid A while it glows very dimly for liquid B. You would conclude that
(i) liquid A is a better conductor than liquid B.
(ii) liquid B is a better conductor than liquid A.
(iii) both liquids are equally conducting.
(iv) conducting properties of liquid cannot be compared in this manner.
Answer: (i) Liquid A is a better conductor than liquid B.
Question 6: Does pure water conduct electricity? If not, what can we do to make it conducting?
Answer: No. Pure water does not conduct electricity. This is because pure water is devoid of any salts. Pure water can conduct electricity when a pinch of common salt is added to it, as salt solution is conducting in nature.
Question 7: In case of a fire, before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Answer: The water usually contains salts and is a good conductor of electricity. To save themselves and others from electric shock and to avoid any short circuit, firemen shut off the main electrical supply for the area.
Question 8: A child staying in a coastal region tests the drinking water and also the seawater with his tester. He finds that the compass needle deflects more in the case of seawater. Can you explain the reason?
Answer: The amount of dissolved salts present in the seawater is more than that of the drinking water. So, the seawater will be a better conductor than the drinking water. That is the reason behind the increased deflection of the needle in the seawater when compared with the drinking water.
Question 9: Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Answer: No. It is not safe to repair electrical appliances outdoors during heavy downpour. This is because rain water contains dissolved salts. Therefore, rain water can conduct electricity. The electrician may get electrical shocks while working outdoors during rain.
Question 10: Paheli had heard that rainwater is as good as distilled water. So she collected some rainwater in a clean glass tumbler and tested it using a tester. To her surprise she found that the compass needle showed deflection. What could be the reasons?
Answer: Air in our surroundings contains gasses, suspended dust particles and pollutants. These particles gets dissolved in rain water and make it good conducting medium of electricity.
Question 11: Prepare a list of objects around you that are electroplated.
Answer: Cold drink cans are tin plated. Artificial Jewellery items are silver or gold-plated. Car bumpers and cycle handles are chromium plated. Metal doors, door handles are zinc plated.
Question 12: The process that you saw in Activity 14.7 is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of battery and why?
Answer: Impure Copper plate should be connected to positive terminal. Pure copper plate should be connected to negative terminal of electrode as copper ions are positively charged and will attract to negative electrode terminal.
NCERT Solutions for Class 8 Science Chapter 14 – A Brief Discussion
CBSE Class 8 Science NCERT Solutions Chapter 14 helps students to clear their doubts and to score good marks in the board exam. All the questions are solved by experts with a detailed explanation that will help students complete their assignments & homework. Having a good grasp over CBSE NCERT Solutions for Class 8 Science will further help the students in their preparation for board exams and other competitive exams such as NTSE, Olympiad, etc.